Topical anaesthetic eye
drops are used extensively in ophthalmology. These agents provide sufficient
corneal and conjunctival anaesthesia for comfortable performance of different
examination techniques like tonometry, biometry, gonioscopy, contact lens
fundoscopy and Nd-YAG laser posterior capsulotomy. Apart from examination
techniques, these agents are used for certain therapeutic purposes as well;
like removal of superficial corneal and conjunctival foreign bodies, removal of
stitches and during surgeries of smaller duration like cataract extraction by
phacoemulsification1.
Topical anaesthetics
used in ophthalmology are tertiary amines linked by either ester or amide bonds
to an aromatic residue. These act by blocking nerve impulse conduction by
decreasing sodium permeability across the cell membranes1. These
agents are weak bases, are more soluble in protonated form and their hydrolysis
is slow in acidic environment so these are bound with hydrochloride1.
Proparacaine is the preferred topical anaesthetic used for ophthalmological
purposes as it causes lesser pain on instillation and longer anesthesia2.
Topical medications are easy to get contaminated and the rate of
contamination is directly related to multiple use and also to duration for
which the drops are being used3,4.
These
drops are used extensively in Ophthalmology outdoor departments and also during
various operative procedures which are known to have potentially vision
threatening infectious complications like endophthalmitis. The rationale of the
study was to check if these drops are a potential cause of endophthalmitis or
other infections of lesser severity like conjunctivitis and keratitis. The
purpose of the study was to
determine the risk of ocular infections due to topical anaesthetic eye drops in
patients one month after opening the bottle.
MATERIALS AND METHODS
This quasi experimental
study was conducted after approval from the ethical and research committee of
DHQ Teaching Hospital, Gujranwala/Gujranwala Medical College. The bottle
contents of a topical formulation containing Proparacaine hydrochloride (0.5%)
as the anaesthetic agent and benzalkonium chloride (0.01%) as a preservative
used in Ophthalmology department of DHQ University teaching hospital,
Gujranwala was tested on daily basis to check if any growth occurs or not. One
bottle of Alcaine® eye drops was labelled as “study case” for this purpose and
drops were instilled daily in eyes of different patients, one from the outdoor
patient department and the other in the operation theater. Thus, 60 patients
were included in our study and they were divided in two groups comprising of 30
patients in each. Group A included those who presented in the outdoor
department and group B comprised of those presenting for various operative
procedures. All patients were adults (18-60 years). Gender of the patients was
not taken as a measure. Patients
were selected through random sampling technique. Those who already had keratitis, conjunctivitis,
corneal opacities, thin corneas and having dry eyes, which were prone to get
infection were excluded from this study. Great care was taken while instilling
eye drops, not to touch tip of the bottle to the ocular surface, adnexa or any
other thing to avoid any possible contamination, which could give false
positive culture results. After instilling drops, the bottle was capped
carefully and tightly before taking any samples for culture growth and stored
in a refrigerator afterwards.
In
the laboratory, sample was applied on blood agar, MacConkey agar and Sabouraud
agar following the conventional techniques. Blood agar and MacConkey agar
plates were incubated at 37° C for 24 hours whereas Sabouraud agar plate was
incubated at 37°C for 24-48 hours to provide adequate time and environment for
bacterial and fungal growth respectively. Samples were cultured daily for 30
days consecutively. All the patients were followed up on day 1, day 7 and day
30 to see if any microbial infection had occurred or not.
RESULTS
More than half (58%)
of the patients in group A presented with complaint of superficial corneal/ conjunctival
foreign body (Figure 1). There were 14 of 30 (47%) patients in group B who were
candidates of cataract extraction by phacoemulsification (Figure 2). There were
37 of 60 (61.6%) patients who complained of transient mild irritation/stinging
after instilling the anaesthetic eye drops while 17 of 60 (28.3%) were noted to
have increased blinking. Others complained of redness and increased lacrimation
(Table 1). All these complaints were short-lived and subsided within an hour.
All
patients were followed up on already defined times and sampling of eye drops
was done daily for 30 days. No signs of bacterial or fungal infection was noted
in any of these patients and no microbial growth was seen on the culture media
we used in our study (Table 2).
Cause of
Presentation in Group A
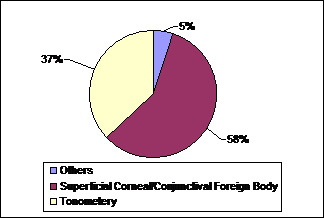
Fig. 1: Cause of
presentation in group A (outdoor patients).
Cause of
Presentation in Group B
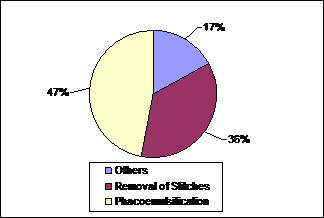
Fig. 2: Cause of
presentation in group B (Operation Theater patients).
Table 1: Complaints while instilling the topical anesthetic
drops.
|
Complaints While Instilling the Drops |
No. of Patients |
Percentage |
|
Irritation/Stinging |
37 |
61.6% |
|
Redness |
5 |
8.3% |
|
Increased lacrimation |
11 |
18.3% |
|
Increased blinking |
17 |
28.3% |
Table 2: Results of growth on different culture media on consecutive 30
days.
|
Culture Medium |
Growth on Day 1 – 30 |
|
MacConkey agar |
No |
|
Blood agar |
No |
|
Sabouraud agar |
No |
DISCUSSION
Topical anesthetics
eye drops are widely used in Ophthalmology for various outdoor and indoor
procedures of short duration; removal of superficial corneal and conjunctival
foreign bodies, tonometry, phacoemulsification etc. These agents provide
sufficient anaesthesia to carry out these procedures under comfortable
circumstances both for the patient and the doctor1. Topical
anaesthesia has largely replaced other techniques of local anesthesia like
retrobulbar/peribulbar anaesthesia being employed during cataract extraction
via phacoemulsification5-7. A study conducted by Rong Han Wu and
co-authors has found topical anaesthesia as a safer alternative to peribulbar/retrobulbar
anaesthesia for patients undergoing pars plana vitrectomy8.
It is a common
practice to use a single bottle of topical formulations in different patients
with different complaints. Asegedech Tsegaw and colleagues found out that
multi-dose eye drops are easily contaminated due to frequent and long-term
usage and may result in inadvertent damage to ocular surfaces by causing
unwanted infections9. Nentwich MM and co-authors conducted a similar
study in Kenya on 101 bottles of multi-use and single-use topical ophthalmic
solutions with similar conclusions10. The rate of contamination is
variable in literature and it ranges from as low as 0.07%11 to as
high as 35.8%12 and is directly related to the increased duration of
use4. A study conducted by Mohammad Reza Fazeli et al. stated that topical anesthetic eye drops were the most
commonly contaminated drops among all other multi-use topical formulations13.
Contamination of the topical formulations is the source of different ocular
infections. The most severe of these is Endophthalmitis, which is infective
inflammation of the intraocular structures and is potentially sight-threatening14.
Topical anaesthetic eye drops are easily available as an over-the-counter
drug and people are likely to get addicted to these medications because of the
temporary relief these agents provide in case of ocular surface irritation.
Some of the medical professionals (the primary care practitioners) also
recommend these as routine medication for this purpose without knowing the
adverse effects these can cause on ocular surface integrity15. Cases
have been reported where the use of topical anaesthetics over long time periods
have been linked to severe damage to eyes in the form toxic keratopathy and
infections16-18.
In a previous study,
it was found that eye drops can be used safely in the hospital settings for up to
2 weeks without being a risk factor for ocular infections4. In our
study, we extended the time period upto 30 days. The reported source of infection in
most of the literature review is contaminated tip 3,9-12 and
contamination of the bottle contents12. In our study, we cultured
only the bottle contents.
Garcia-Arumi and co-authors have conducted a similar retrospective study
on patients undergoing cataract surgery and have found topical anesthesia to be
related to postoperative endophthalmitis with an odds ratio [OR] of 11.8 and
95% confidence interval [CI]19. Hou-Chuan Lai and co-authors have
also conducted a retrospective study comparing different types of anaesthesia
for intraocular surgeries and found out that the rate of postoperative
endophthalmitis after phacoemulsification is significantly higher for topical
anaesthesia as compared to general anaesthesia (0.083:0)20.
The results of our study are in contrast to
all the similar studies conducted in the past as no growth was observed in the
bottle contents and no signs of infection was noted in the eyes of patients in whom
the drops were instilled. The limitation
of the study is small sample size and a similar study is needed to be conducted
on a larger scale considering other risk factors like time of presentation to
the doctor, duration of surgery and the frequency and technique of instillation
of eye drops to carry out different procedures with full convenience. We also
recommend to carry out this research on a large scale so as to get more
references in this regard.
CONCLUSION
On
the basis of results of our study, we conclude that Alcaine® eye drops remain
sterile for 1 month and can be used safely in different patients for this
duration without any risk of causing bacterial or fungal infections, if care is
taken while instilling the drops as previous studies have documented tip
contamination as a source of ocular infections. This is also beneficial from
economical point of view.
Conflict of Interest
The
authors have no conflicts of interest to disclose regarding this study.
REFERENCES
1. Kumar
M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015; 31 (4): 450–456.
Doi:10.4103/0970-9185.169049.
2. Bartfield JM, Holmes TJ, Raccio-Robak N. A
comparison of proparacaine and tetracaine eye anesthetics. Acad Emerg Med.
1994; 1 (4): 364–367.
3. Tamrat L, Gelaw Y, Beyene G, Gize A. Microbial
Contamination and Antimicrobial Resistance in Use of Ophthalmic Solutions at
the Department of Ophthalmology, Jimma University Specialized Hospital, Southwest
Ethiopia. Can J Infect Dis Med Microbiol. 2019, Article ID 5372530, 8 pages,
4. Livingstone DJ, Hanlon GW, Dyke S.
Evaluation of an extended period of use for preserved eye drops in hospital
practice. Br J Ophthalmol. 1998; 82 (5): 473–475. Doi:10.1136/bjo.82.5.473.
5. Guay J, Sales K. Sub‐Tenon's anaesthesia versus topical anaesthesia
for cataract surgery. Cochrane Database of Systematic Reviews, 2015, Issue 8.
Art. No.: CD006291. DOI:10.1002/14651858.CD006291.pub3.
6. Jacobi PC, Dietlein TS, Jacobi FK. A
Comparative Study of Topical vs. Retrobulbar Anesthesia in Complicated Cataract
Surgery. Arch Ophthalmol. 2000; 118 (8): 1037–1043. Doi:10.1001/archopht.118.8.1037.
7. Malik A. Efficacy and Performance of
Various Local Anesthesia Modalities for Cataract Surgery. J Clinic Experiment
Ophthalmol. 2013; S1: 007.
Doi:10.4172/2155-9570.S1-007
8. Wu RH, Zhang R, Lin Z, Liang QH, Moonasar
N. A comparison between topical and retrobulbar anesthesia in 27-gauge
vitrectomy for vitreous floaters: a randomized controlled trial. BMC
Ophthalmol. 2018; 18 (1): 164.
9. Tsegaw A, Abula T, Assefa Y. Bacterial
Contamination of Multi-dose Eye Drops at Ophthalmology Department, University
of Gondar, Northwest Ethiopia. Middle East Afr J Ophthalmol. 2017; 24 (2): 81–86.
Doi:10.4103/meajo.MEAJO_308_16.
10. Nentwich MM, Kollmann KH, Meshack J, Ilako
DR, Schaller UC. Microbial contamination of multi-use ophthalmic solutions
in Kenya. Br J Ophthalmol. 2007; 91 (10): 1265–1268. Doi:10.1136/bjo.2007.116897.
11. Wessels IF, Bekendam P, Calvin WS,
Zimmerman J. Open drops in ophthalmology offices: expiration and
contamination. Ophthalmic Surg Lasers 1999; 30: 540–546.
12. Brudieu E, Duc DL, Masella JJ,
Croize J, Valence B, Meylan I, et al. Bacterial contamination of multi‐dose ocular solutions. A prospective
study at the Grenoble Teaching Hospital. Pathol Biol (Paris), 1999; 47: 1065–1070.
13. Fazeli MR, Nejad HB, Mehrgan H, Elahian L,
“Microbial contamination of preserved ophthalmic drops in outpatient
departments: possibility of an extended period of use,” DARU J Pharm. Sci.2004;
12 (4): 151–155.
14. Durand ML. Bacterial and Fungal
Endophthalmitis. Clin Microbiol Rev. 2017; 30 (3): 597–613.
Doi:10.1128/CMR.00113-16
15. Erdem E, Undar IH, Esen E, Yar K, Yagmur M, Ersoz
R. Topical anesthetic eye drops abuse: are we aware of the danger? Cutan Ocu
Toxicol. 2013; 32 (3): 189-193.
16. Aksoy A, Başkan AM, Aslan L, Aslankurt
M. Topical proparacaine abuse resulting in evisceration. BMJ Case Rep.
2013; 2013: bcr2013009539. Published 2013 Apr 22. Doi:10.1136/bcr-2013-009539
17. Tok OY, Tok L, Atay IM, Argun TC, Demirci
N, Gunes A. Toxic keratopathy associated with abuse of topical anesthetics
and amniotic membrane transplantation for treatment. Int J Ophthalmol. 2015; 8 (5):
938–944. Doi:10.3980/j.issn.2222-3959.2015.05.15
18. Kintner JC, Grossniklaus HE, Lass JH,
Jacobs G. Infectious crystalline keratopathy associated with topical
anesthetic abuse. Cornea. 1990; 9 (1): 77–80.
19. Garcia-Arumi
J, Fonollosa A, Sararols L, Fina F,
Martínez-Castillo V, Boixadera A et al. Topical anesthesia: possible risk
factor for endophthalmitis after cataract extraction. J Cataract Refract Surg.
2007; 33(6): 989-92.
20. Lai HC, Tseng WC, Pao SI, Wong CS, Huang
RC, Chan WH et al. Relationship between anesthesia and postoperative
endophthalmitis: a retrospective study. Medicine, 2017; 96: e6455.